Li-Ping Qu (Chair) , Gerhard Franz (Co-Chair) and Abraham Chan (Co-Chair)
The objective of this interest group is to support scientists and industries from non-European countries to discuss and to understand the basic rules for the regulation and implementation of herbal medicinal products in the EU. To fulfil the acceptance of non-European herbal medicinal products, quality, safety and efficacy are the three important pillars for assessment of herbal medicinal products. We are aiming to organise workshops and panel discussions with experts and scientists/industries active in this field, to promote products that fulfil the EU regulation to enter the EU market.
Chinese herbal products (CHPs) are defined as Chinese herbal drugs, Chinese herbal drug extracts, Chinese herbal medicinal products and traditional Chinese medicine granules (TCM granules). The use of CHPs should be under the guidance of Traditional Chinese medicine (TCM) theories. With the worldwide application of TCM, CHPs have entered the international market quickly. Meanwhile, their scientific evidence, safety issues/possible toxicities and quality issues are on a solid base and have also gained a lot of attention. From EU regulatory perspective, it plays one of the key roles for CHMs to be accepted and implemented by European member states.
The European medicinal regulatory system is based on the network of regulatory authorities from the European Economic Area countries: the European Commission, the European Medicines Agency (EMA, https://www.ema.europa.eu/en) and the European Directorate for the Quality of Medicines and Health Care (EDQM, https://www.edqm.eu), making the EU regulatory system unique in the world. The EDQM develops the European Pharmacopeia (Ph. Eur), which covers the quality requirements for a wide range of pharmaceutical substances, including herbal drugs and extracts. In this system, the EMA and the EDQM play core roles in the medicinal registrations and quality managements (see figure below).
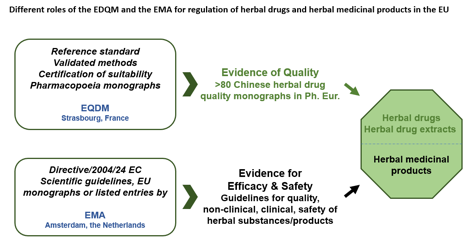
Figure: Different roles of the EDQM and the EMA for regulation of herbal medicinal products in the EU.
Within the EU, the Ph. Eur. under the management of the EQDM is a legally binding document for quality standards for medicinal products. The EMA is mainly responsible for the proof of efficacy and safety of medicinal products. The combination of both institutions plays central roles in medicinal products. Two different terms are actually used: Herbal drug (Ph. Eur.) = Herbal substance (EMA); herbal medicinal product (Ph. Eur. and EMA). This figure is extracted from an article published in Chinese medicine (2022) 17:29 by Wang et al.
![]()
